Mastering Basis Sets in Theoretical Chemistry: Physical Meaning, Types, Applications, and BSSE Correction
Basis Set in Theoretical Chemistry: An Introduction
In theoretical
chemistry, the concept of a basis set plays a fundamental role in the
calculation of molecular properties. A basis set is a collection of functions
used to approximate the wavefunction of a molecule. The wavefunction represents
the quantum mechanical state of a molecule, and its calculation is the
foundation for the prediction of molecular properties such as bond lengths,
bond angles, and energies. The choice of basis set significantly affects the
accuracy and computational cost of the calculation. Therefore, selecting the
most suitable basis set is critical for obtaining reliable and accurate
results.
We will be
looking at...
- ·
why we use basis sets.
- ·
the physical meaning of basis
sets.
- ·
why to use STOs and GTOs.
- ·
how we use basis sets.
- ·
basis set notation.
- ·
choosing a basis set
- ·
the quality of basis sets.
- BSSE
- ·
methods vs basis set’ll look at…
Basis Set?
·
Set of mathematical functions
(basis functions) which represents the electronic wavefunction in HF/DFT.
- ·
Help in solving the Schrödinger
equation.
- ·
Linear combination of basis
function approximate total electronic wavefunction (Ψ) and molecular orbitals.
- ·
Types of used atomic orbitals :
Gaussian-type orbitals (GTOs), Slater-type orbitals (STOs) and Numerical atomic
orbitals
- · Most often people uses GTOs
Why Basis Set?
We want one or both of the following :-
Ø The electronic energy of molecule.
Ø The wavefunction for molecule so that we can calculate other
properties.
We satisfy our necessity by solving the stationary state
Schrödinger equation.
ĤΨ = EΨ
·
Solving the Schrödinger equation
to obtain Ψ of hydrogenic atom is easy.
·
But for more than two particles
we are forced to make guesses for Ψ.
·
One guess is to use functions
that are similar to the formulae obtained already (s, p, d, f etc. atomic
orbitals (AO’s)).
·
Basis sets, very loosely, as
sets of functions like s, p, d, f, etc. that will be used to describe the
behavior of electrons in all systems whether they be hydrogenic or not.
How to
Understand Basis Set
Physical
Meaning and Use of basis set
Ø Several (usually) basis functions describe the electron
distribution around an atom.
Ø Combining atomic basis functions yields electron distribution in
whole molecule.
Ø Basis sets are used to approximate Ψ.
Ø The bigger and better the basis set, the closer we get to Ψ, and hence E.
Ø Nowadays, almost everyone utilizes gaussian functions in basis sets.
Ø One or more gaussian-type functions are used for each AO in each
atom in the molecule of interest.
Slater functions are used in semiempirical calculations, like EHM and other semiempirical methods. Modern molecular ab initio program employ Gaussian functions.
There are
various types of basis sets, and each has its own advantages and disadvantages.
Below are the most commonly used types of basis sets in theoretical chemistry.
Minimal Basis
Set:
A minimal basis
set consists of a small number of functions, usually one per atom. These
functions are generally atomic orbitals such as 1s, 2s, 2p, and so on. The
advantage of a minimal basis set is that it is computationally efficient,
making it suitable for calculations on large molecules. However, the accuracy
of the results obtained from a minimal basis set is often limited.
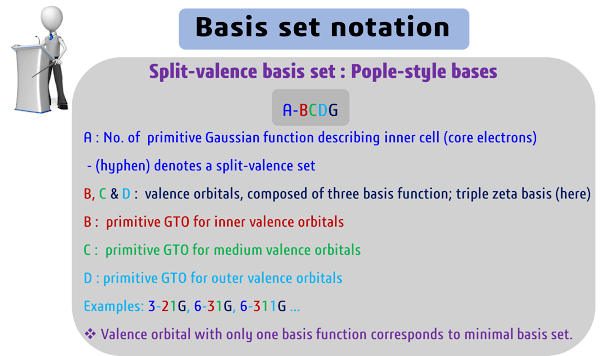
Split Valence
Basis Set:
A split valence
basis set consists of two sets of functions, a core set, and a valence set. The
core set contains the functions that are shared by all atoms in the molecule,
while the valence set contains functions that are unique to each atom. The
advantage of a split valence basis set is that it provides a more accurate
representation of the electron density of the molecule than a minimal basis
set.
Polarized Basis
Set:
A polarized
basis set includes additional functions to account for the polarization of
electron density around an atom caused by the presence of nearby atoms. These
functions are generally more diffuse than those in a minimal or split valence
basis set. The inclusion of polarization functions improves the accuracy of the
results and is particularly important for calculations involving anions or
cations.
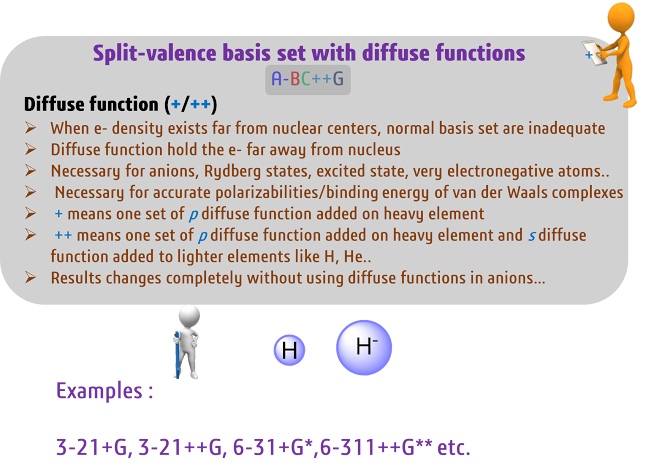
Diffuse Basis
Set:
A diffuse basis
set includes functions that are more diffuse than those in a polarized basis
set. These functions are used to describe the electron density in the outer
regions of the molecule, such as in the case of anions or cations. A diffuse
basis set is particularly useful for calculations that involve the prediction
of properties such as dipole moments and electron affinities.
Pseudopotential
Basis Set:
A
pseudopotential basis set is used to represent the electron density of an atom
by using pseudopotentials instead of the actual electron wavefunctions. The
advantage of a pseudopotential basis set is that it significantly reduces the
computational cost of the calculation, particularly for heavy elements.
However, the accuracy of the results obtained from a pseudopotential basis set
is generally lower than that of a full-electron basis set.
Conclusion
In summary, the choice of basis set is an important consideration in theoretical chemistry calculations. The selection of an appropriate basis set depends on the level of accuracy required, the size of the molecule being studied, and the computational resources available. Each type of basis set has its own advantages and disadvantages, and selecting the most suitable basis set for a given calculation requires careful consideration.





















Comments
Post a Comment