Ionic bonding is a type of chemical bonding that involves the transfer of electrons from one atom to another. This type of bonding results in the formation of ions, which are atoms or molecules that have a positive or negative charge. Ionic bonds are characterized by their strong electrostatic attraction, high melting and boiling points, and low electrical conductivity. In this blog, we will explore ionic bonding in detail, including its definition, properties, and examples.
Definition of Ionic Bonding
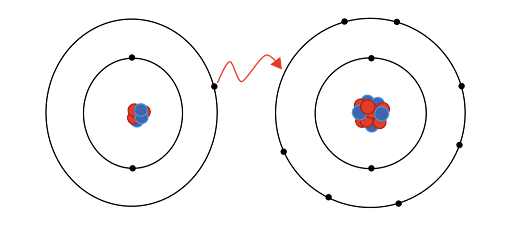
Ionic bonding refers to the transfer of
electrons from one atom to another, resulting in the formation of ions with a
positive or negative charge. The positive ion, called a cation, is formed by
the loss of electrons, while the negative ion, called an anion, is formed by
the gain of electrons. The cation and anion are then attracted to each other by
the electrostatic forces of attraction, forming an ionic bond.
Properties of Ionic Bonding
High Melting and Boiling Points: Ionic bonds
are strong electrostatic forces of attraction between the cation and anion.
These strong bonds result in high melting and boiling points, as a large amount
of energy is required to break the bonds and separate the ions.
Low Electrical Conductivity: Ionic compounds
are poor conductors of electricity in their solid state because the ions are
held rigidly in place by the ionic bonds. However, they can conduct electricity
when melted or dissolved in a polar solvent, such as water, because the ions
are free to move and conduct electric current.
Brittle Nature: Ionic compounds are
typically brittle and break easily when subjected to stress. This is because
the ionic bonds are rigid and cannot bend or deform to absorb the stress,
causing the compound to break.
High Lattice Energy: Ionic compounds have
high lattice energies, which is the energy required to separate the ions in the
compound. This high energy is due to the strong electrostatic attraction
between the cations and anions.
Examples of Ionic Bonding
Sodium Chloride (NaCl): Sodium chloride is a
common ionic compound formed from the transfer of an electron from sodium to
chlorine. The sodium becomes a positively charged cation, while the chlorine
becomes a negatively charged anion, and they are attracted to each other by the
electrostatic forces of attraction to form an ionic bond.
Calcium Oxide (CaO): Calcium oxide is
another example of an ionic compound, formed from the transfer of electrons
from calcium to oxygen. The calcium becomes a positively charged cation, while
the oxygen becomes a negatively charged anion, and they are attracted to each
other by the electrostatic forces of attraction to form an ionic bond.
Magnesium Oxide (MgO): Magnesium oxide is an
ionic compound formed from the transfer of electrons from magnesium to oxygen.
The magnesium becomes a positively charged cation, while the oxygen becomes a
negatively charged anion, and they are attracted to each other by the
electrostatic forces of attraction to form an ionic bond.
Conclusion
Ionic bonding is a type of chemical bonding
that involves the transfer of electrons from one atom to another, resulting in
the formation of ions with a positive or negative charge. Ionic bonds are
characterized by their strong electrostatic attraction, high melting and
boiling points, and low electrical conductivity. Understanding ionic bonding is
important for understanding the properties and behavior of ionic compounds, as
well as for designing new materials and compounds.

Comments
Post a Comment